|
Solid Polymer electrolytes
This promising lithium rechargeable technology is using a polymer electrolyte in a solid state cell in which a polymer
electrolyte is sandwiched between a lithium metal film and a metal film. By dissolving lithium, not into a liquid
electrolyte but into a really thin polymer (plastic), a high-power battery is realized that is light, yet durable. The
laminate construction of such cells offer flexibility of shape and size, which is advantageous for portable power source
applications. However, at the present time, the conductivity of these batteries is very low at room temperature, compared
with those of liquid electrolytes: these batteries are normally operated in the 60-120oC range. Research is being aimed
at increasing conductivity through the use of plasticizers and new polymers. This new technology offers the potential
of future low manufacturing costs. It is environmentally benign, it avoids electrolyte leakage to damage electronic
components, and can fit any casing shape. It can be used either as a rechargeable system for training or peacetime
exercises, or as a primary battery in emergency . Dissolution of the polymer into these melt-glass-electrolytes produces
a rubbery version of a glassy electrolyte with a thousand-fold increase in lithium ion mobility. A way has been opened
to a new generation of lithium batteries with the prospect of a high power density application. Much work remains to be
done before this discovery can be fully exploited. Polyethylene oxide (PEO) based solid polymer electrolytes comprised
of glymes, represented by formula CH3-(O-CH2-CH2)-OCH3 complexed with lithium salts of weakly coordinated ions such as
CF3SO3- or (CF3SO3)2N- exhibit high ionic conductivity, facilitate their use in solid state batteries and electrochromic
devices. The factors underlying this ion transport phenomenon include (i) Cation-oligomer interactions (ii) Ionic association
(formation of ion triplets or ion pairs) and (iii) Polymer segmental motion. Our work focusses on the molecular level
understanding of these factors utilizing the ab initio and Density functional calculations. Also topography of 3D
scalar functions like Molecular Electrostatic Potential and Molecular Electron Density which provides signature of
bonding can be explored to have in site of Electronic structure. |
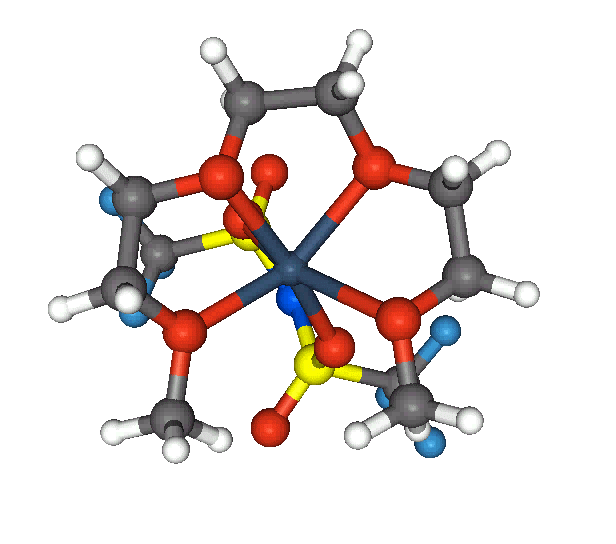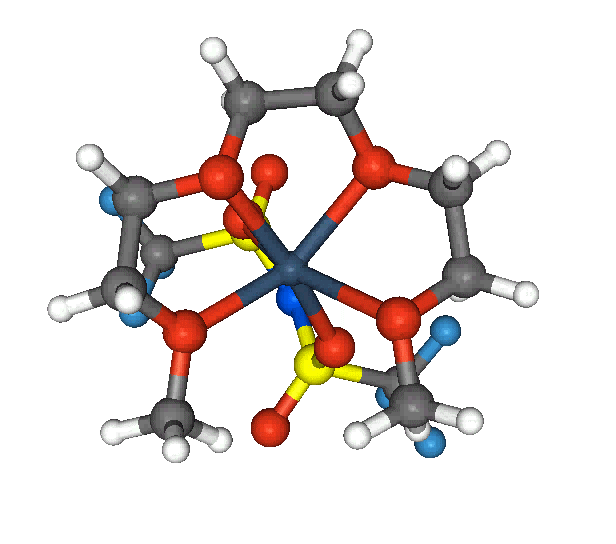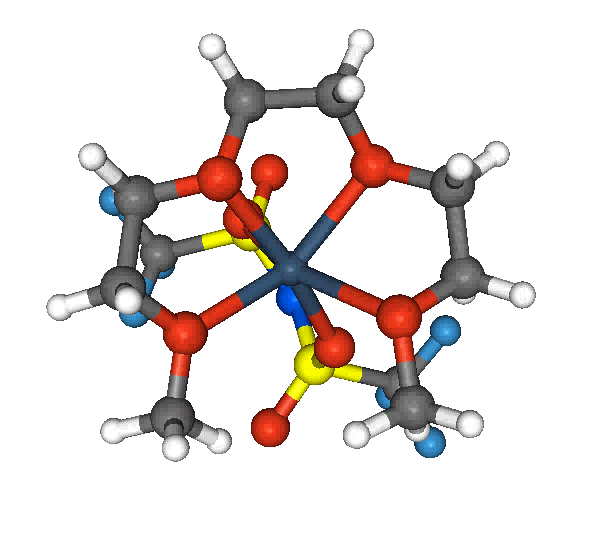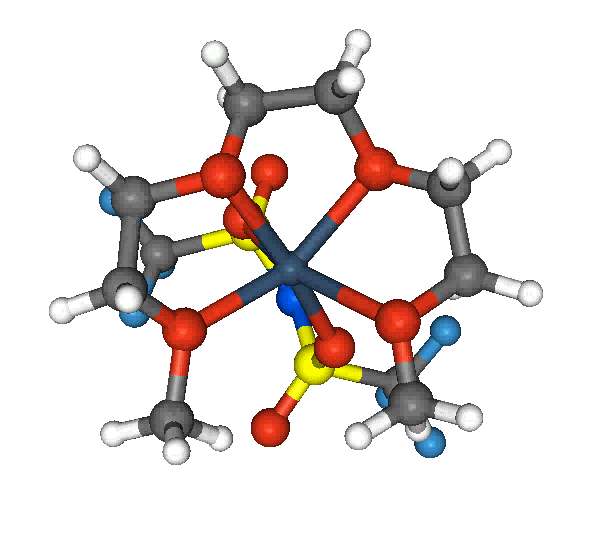
Fig. 1
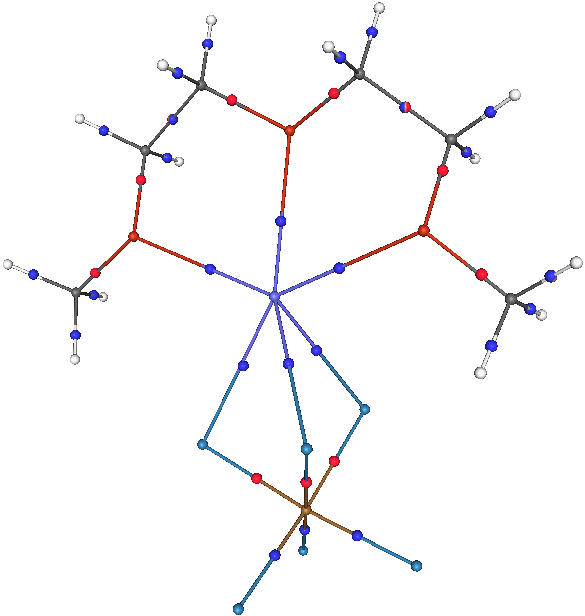
Fig. 2
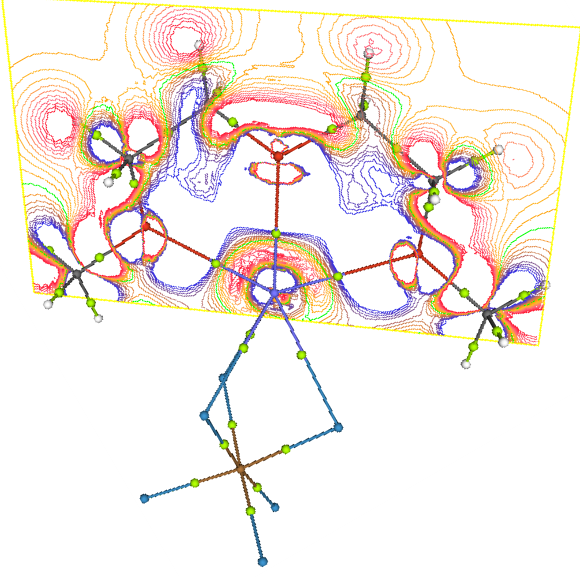
Fig. 3
|




